For more than a decade, Paul Ridker kept the reviewer comments of one of his first grant proposals taped to the wall of his office. The comments were scathing, calling his ideas “naive” and worse; to Ridker, the document represented the difficulty of clearing the blockages caused by scientific conventional wisdom.
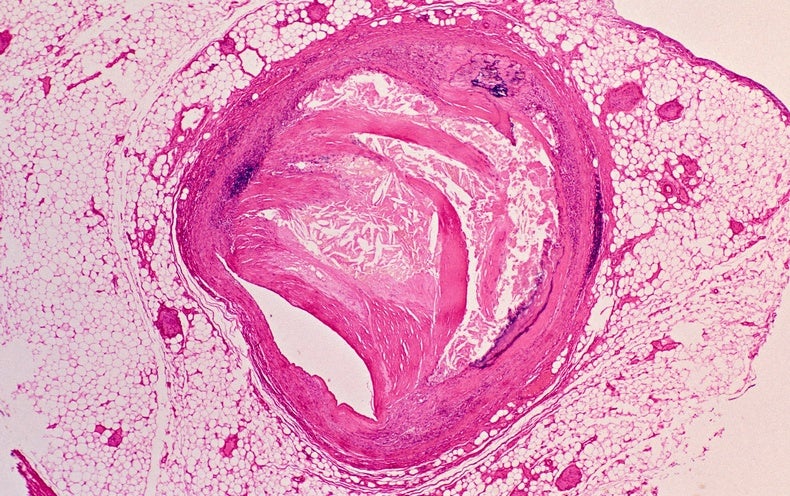
“This was against all dogma at the time,” Ridker, a cardiologist and epidemiologist at Brigham and Women’s Hospital in Boston, Massachusetts, says of his proposal to search for links between inflammation and cardiovascular disease. In the early 1990s, most researchers believed that atherosclerosis—the build-up of fatty deposits known as plaques in blood vessels that can contribute to heart attacks and strokes—was driven more or less exclusively by excess cholesterol in the blood.
Ridker’s implication of inflammation was so out of the mainstream that, even after he and his team found a way to collect promising preliminary data without the funding, their proposal was rejected a second time (it was eventually funded). In 1997, they reported that middle-aged men with high levels of an inflammatory marker known as C-reactive protein (CRP) were at increased risk of heart attack and stroke, regardless of their blood cholesterol level1.
The paper was a turning point. The idea that cardiovascular disease is an inflammatory condition is broadly accepted today. In the past several years, a handful of large clinical trials has refined the understanding of the pathways involved and brought new anti-inflammatory therapies for cardiovascular disease close to use in the clinic.
The trials “have been extremely important in providing proof of concept that inflammation is not only a risk factor and a pathogenic mechanism, but actually a treatable component to the disease process”, says Göran Hansson, a cardiologist at the Karolinska Institute, Stockholm.
But turning that proof of concept into widespread practice will require researchers to pinpoint where in the body the inflammation that contributes to cardiovascular disease occurs, and how the cells and molecules involved differ from one person to the next, says Robin Choudhury, a cardiologist at the University of Oxford, UK. “It’s a question of precision,” he says. “The term inflammation has become overly loosely applied.”
Markers and mechanisms
Even in the early 1990s, evidence hinted that cholesterol wasn’t the whole story. After all, almost half of heart attacks and strokes in the United States occurred in people who did not have high cholesterol. About one-quarter were in people who had no known cardiovascular risk factor. And the connection to inflammation was not entirely new, either. In the mid-1880s, German pathologist Rudolf Virchow recognized inflammatory cells in atherosclerotic plaques. Theories linked to inflammation failed to gain traction, however, because “there was almost no clinical data”, says Ridker.
Ridker’s study helped to fill in the missing piece of the puzzle. But he wasn’t the only heretic thinking about inflammation in the 1990s. Researchers led by Mark Pepys at University College London also linked CRP to cardiovascular disease2, as well as a related protein called serum amyloid A, adding credibility to the emerging inflammation story.
CRP remains the primary way to measure cardiovascular-disease-related inflammation, although it doesn’t have a causal role in atherosclerosis itself. Instead, CRP is produced by the liver as a signal of inflammation elsewhere in the body. CRP is tied, in particular, to a pathway that includes the signalling molecules interleukin (IL)-1β, IL-18 and IL-6, which, in the past few years, have emerged as key players in atherosclerosis.
But cell culture and animal studies have revealed a variety of other cells and molecules that contribute to the process. When cholesterol in the form of low-density lipoprotein (LDL) sticks to or gets inside the wall of an artery, it triggers the release of pro-inflammatory signalling molecules. These molecules attract white blood cells known as monocytes, which take up residence in the artery wall. There, the monocytes become lipid-laden foam cells that send out more signalling molecules to keep inflammation going. Immune cells called T cells also become activated by pieces of degraded LDL, and can contribute to triggering a heart attack.
Such findings suggest that cardiovascular disease involves an interplay between cholesterol and inflammation. “You need a little bit of fuel”—in this case, cholesterol—to get an atherosclerotic plaque started, says cardiologist Erin Michos at Johns Hopkins University, Baltimore, Maryland. But it is the subsequent inflammation that causes atherosclerosis to take hold, she adds. “Inflammation is definitely the fire.”
Trial, trial again
By the early 2000s, growing evidence that statins—a class of lipid-lowering drugs given to people with cardiovascular disease—also have inflammation calming effects provided an opportunity to test whether treating inflammation could help keep atherosclerosis in check.
Ridker and his colleagues recruited almost 18,000 people with low cholesterol but elevated CRP levels and randomly assigned half of them to take a statin drug and the other half a placebo3. In this trial—called JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)—those who took the drug saw a 44% reduction in their risk of a heart attack, stroke or other cardiovascular emergency. The JUPITER results, reported in 2008, helped to change clinical practice—especially for the management of people who have not yet had a heart attack or stroke. In individuals who are at intermediate or uncertain risk of cardiovascular disease, elevated CRP can signal the need for statin treatment, says Michos.
Still, the JUPITER study didn’t definitively answer the question of whether the statins helped people by reducing inflammation or by lowering lipids. “The only way to actually test the inflammation hypothesis would be to give them some sort of intervention that was purely inflammation inhibiting,” Ridker says.
To do that, Ridker and his team launched another study4, called CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study). This tested whether canakinumab—a monoclonal antibody made by pharmaceutical company Novartis in Basel, Switzerland, that inhibits IL-1β but does not have any effect on lipids—could reduce the risk of heart attacks and strokes in around 10,000 people with elevated CRP who had already had a heart attack. Those who received the drug in addition to standard therapy had a 15–17% lower chance of having a heart attack or stroke, or of dying from a cardiovascular event than people who received a placebo, the team reported in 2017.
The findings were widely hailed as proof of concept for anti-inflammatory therapy in cardiovascular disease. But some view the results more cautiously. The modest benefit seen in the trial is a signal that the treatment might not have been targeted at the right group of people, says Choudhury. Clinical trials “don’t come close to really personalizing or precision guiding those therapies”, he says, adding that more detailed studies, such as of gene expression in immune cells sampled from atherosclerotic plaques, will be necessary to work this out.
Like most monoclonal-antibody drugs, canakinumab is very expensive—and after facing regulatory hurdles from US and European agencies, Novartis opted not to continue seeking approval for use in cardiovascular disease. That deflated some enthusiasm about anti-inflammatory therapies.
So too did negative results from a second trial, known as CIRT (Cardiovascular Inflammation Reduction Trial). In this trial5, Ridker and his colleagues tested whether methotrexate—a well-established, inexpensive immune-suppressing drug—would reduce heart attacks, strokes and cardiovascular death in about 4,800 people with coronary artery disease and type 2 diabetes or metabolic syndrome. But the drug had no effect on these outcomes, nor did it reduce levels of IL-1β, IL-6 or CRP.
However, Ridker thinks that the negative results nonetheless provide useful information. At the time CANTOS and CIRT were designed, “we weren’t certain which of various different targeted inflammatory pathways were crucial”, he says. The conspicuous absence of any effect on IL-1β, IL-6 or CRP in the failed CIRT indicates that anti-inflammatory drugs for atherosclerosis need to target this pathway, as canakinumab does.
Back to basics
Researchers are getting closer to reaping the therapeutic fruits of the many inflammation studies. Promising results over the past year from two placebo-controlled trials of colchicine, a drug used to treat arthritic conditions such as gout, “provide independent confirmation of what we saw in CANTOS”, Ridker says. Colchicine targets the same inflammatory pathway as canakinumab.
“We had a drug that was known to be safe, that has been around for more than a century, that was widely available, orally administered and cheap. So for us, it was the ideal agent to test,” says Jean-Claude Tardif, a cardiologist at the Montreal Heart Institute in Canada. Tardif led one of the two colchicine trials6, known as COLCOT (Colchicine Cardiovascular Outcomes Trial), involving 4,745 participants.
In COLCOT, colchicine resulted in a 23% reduction in future cardiac events or death; in a slightly larger trial7 called LoDoCo2 (Low-dose Colchicine 2), the reduction was 31%. In fact, a cost-effectiveness analysis of the COLCOT data showed that the drug was an exceedingly good deal. “Anytime you give colchicine to a patient who has recently had a heart attack, you actually decrease overall costs in the health-care system,” Tardif says.
Some cardiologists are already beginning to prescribe colchicine for certain people with cardiovascular disease. But researchers are also launching additional trials of colchicine and other anti-inflammatory drugs. Tardif is planning a study that will investigate the role of colchicine earlier in the disease process, testing whether the drug prevents a first heart attack in 10,000 people with type 2 diabetes.
At the American College of Cardiology meeting, held virtually in May, Ridker announced a phase III trial of ziltivekimab, a monoclonal antibody that targets IL-6; it is the first drug in its class to be developed specifically for cardiovascular disease. The trial will test ziltivekimab in 6,000 people with chronic kidney disease, a population that is at elevated risk of dying from heart attacks and strokes but that cannot take colchicine, which is processed by the kidneys.
Thirty years on from his radical proposal, Ridker still has a propensity to think big. He foresees a kind of grand unification approach emerging in which both lipids and inflammation are seen as essential elements to treating heart disease. “In the future, we’re going to bring these two things back together,” he says. “My belief is that the sum will be greater than the parts. If we could figure out an inexpensive, safe way to dramatically lower cholesterol and dramatically lower inflammation earlier in life, maybe we could actually eliminate this disease.”
This article is part of Nature Outlook: Heart health, an editorially independent supplement produced with the financial support of third parties. About this content.
References
-
Ridker, P. M. et al. N. Engl. J. Med. 336, 973–979 (1997).
-
Liuzzo, G. et al. N. Engl. J. Med. 331, 417–424 (1994).
-
Ridker, P. M. et al. N. Engl. J. Med. 359, 2195–2207 (2008).
-
Ridker, P. M. et al. N. Engl. J. Med. 377, 1119–1131 (2017).
-
Ridker, P. M. et al. N. Engl. J. Med. 380, 752–762 (2019).
-
Tardif, J.-C. et al. N. Engl. J. Med. 381, 2497–2505 (2019).
-
Nidorf, S. M. et al. N. Engl. J. Med. 383, 1838–1847 (2020).