Editor’s Note (5/3/23): The U.S. Food and Drug Administration has approved GSK’s respiratory syncytial virus (RSV) vaccine for adults age 60 and older—the first approved vaccine for this disease. The road to its development was long and bumpy but ultimately successful, as detailed in this story published on March 20.
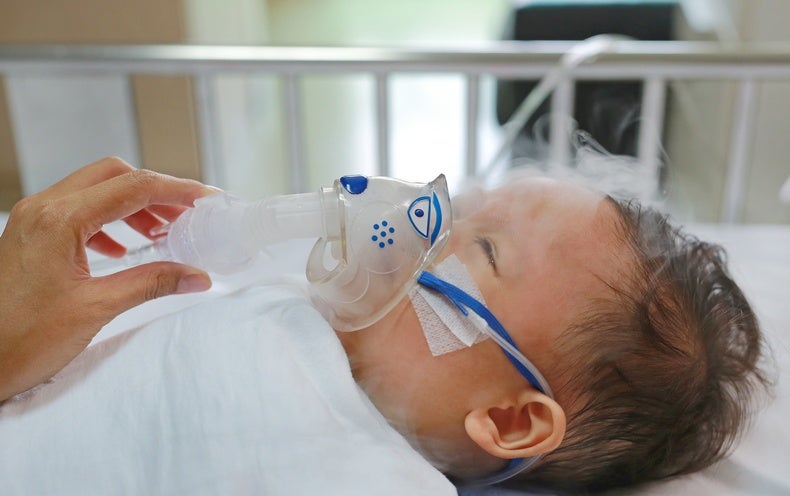
Megan Smith was especially conscious of the threat COVID posed to her six-week-old daughter in October 2021. The now 36-year-old from Buffalo, N.Y., was “doing everything we could to protect her,” Smith says. Ultimately, though, it wasn’t COVID that sent her baby to the hospital but a more mundane virus that infects nearly everyone by their second birthday: respiratory syncytial virus (RSV).
Smith’s daughter required hospitalization and intubation from this respiratory virus, which infects the nose, throat, lungs and breathing passages. “I was not prepared for this in any sort of way,” says Smith, who was startled to learn no treatments for RSV exist besides supportive care and oxygen. Her daughter recovered, but Smith was left wishing a vaccine could have saved her that stress and heartache.
Scientists have been working on an RSV vaccine since soon after the virus was discovered in 1956, but some disastrous clinical trials in the 1960s and dozens of failed attempts at vaccine development stymied progress—until recently. The U.S. Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted on February 28 and March 1 to recommend FDA approval for two RSV vaccines, one made by GSK and one by Pfizer, for adults aged 60 and older. The FDA, which typically follows VRBPAC’s recommendations, is expected to issue a decision by May.
Two other new RSV vaccines—Moderna’s for older adults and Pfizer’s for pregnant people—are headed for FDA consideration this year. Regulators could also approve nirsevimab, a new long-acting monoclonal antibody that offers protection similar to a vaccine in infants for up to five months, which is about the length of a typical RSV season. Nirsevimab is already approved in Europe.
This breakthrough in RSV vaccine research happened once researchers solved a 50-year-old mystery about the virus by examining the shape of its proteins. The process has ushered in a new era of vaccine development using protein-structure-based vaccine design—the same approach that enabled the rapid development of a COVID vaccine.
A Tragic History
For most people, RSV is little more than a troublesome cold with symptoms that include coughing, sneezing, wheezing, runny nose and fever. But it became an early vaccine target because of the danger it poses to young infants, older adults, immunocompromised people and those with a chronic heart or lung disease. An estimated 58,000 children and 177,000 older adults are hospitalized with RSV each year, resulting in the deaths of 100 to 500 children and approximately 14,000 older adults. The disease, which costs the U.S. more than $1 billion each year, is the leading cause of hospitalization in infants.
A decade after the virus’s discovery, in 1966, four clinical trials tested an inactivated virus vaccine in children who had never encountered RSV before. To the scientists’ horror, in one of the studies, 80 percent of the vaccinated children were hospitalized when they later contracted the virus itself, and two toddlers—a 14-month-old and a 16-month-old—died. Typical hospitalization rates for RSV are in the single digits, says Ruth Karron, a pediatrician and director of the Johns Hopkins Vaccine Initiative.* While otherwise healthy children do sometimes die from RSV, it is most likely to occur in the first six months of life.
“As you can imagine, this sort of stopped vaccine development for a very long time,” Karron says. “You took a pathogen that, even then, didn’t kill that many children, and it killed children.”
For the next two decades, RSV vaccine progress stagnated. Researchers needed to know what had gone so wrong in the 1960s. The mystery wasn’t solved until 2008, when Fernando P. Polack, founder of the Infant Foundation in Argentina, and his team at Johns Hopkins University published a study in Nature Medicine describing how the antibodies produced by the vaccinated children’s immune systems did not bind strongly enough to the virus. Instead the antibodies attracted dead viruses and sparked a dangerous cascade of abnormal immune responses that caused severe inflammation in the lungs, making the children sicker than they would have been with no preexisting antibodies.
But a big question remained: Why didn’t those antibodies bind adequately to the virus? Later that same year a serendipitous meeting would lead to the final pieces of the puzzle necessary to make RSV vaccines a reality.
A Tale of Two Protein Shapes
In June 2008 Jason McLellan, now a molecular biologist at the University of Texas at Austin, had just completed his Ph.D. at Johns Hopkins and begun a postdoctoral fellowship at the National Institutes of Health Vaccine Research Center, where he met Barney Graham, now a senior adviser for global health equity at Morehouse School of Medicine. Graham had devoted his career to studying RSV and learned that McLellan, who specialized in mapping the atomic structure of proteins, was interested in working on something “a little off the radar,” Graham says. “Well, we have no structural information on RSV yet,” he told McLellan. Graham was particularly interested in the F protein, the main target for RSV vaccine development. The F protein is an antigen, the part of a pathogen that the immune system recognizes as a threat and makes antibodies against.
The idea piqued McLellan’s interest. “It became clear that RSV was one of the major childhood pathogens for which we didn’t have a vaccine, so working on a vaccine that can help save the lives of babies and young children was very motivating,” he says.
The pair’s goal—discovering the F protein’s structure—would become the key to creating a successful vaccine. But the F protein isn’t stable: when it fuses with a cell, allowing the virus to enter and hijack the cell to reproduce, it changes shape. Antibodies against the postfusion shape—the ones produced by the immune systems of the children in the 1960s trials—don’t neutralize the circulating form of the virus that well before it binds to cells. But if a vaccine could induce antibodies against its prefusion form, they might bind properly with the active form of virus. The trick was to figure out what that prefusion protein looked like and how to lock it into that shape.
By 2010 McLellan had determined the structure of the postfusion protein using a structural imaging technique called x-ray crystallography. He then turned to the prefusion structure so he and his team could compare the prefusion and postfusion structures and figure out how to keep it from shifting forms. Collaborating with researchers in China, McLellan and Graham tested more than 2,000 mouse antibodies until they found one that effectively neutralized, or deactivated, the prefusion F protein without binding to the postfusion one (thereby eliminating the risk of the hyperinflammatory response caused by the RSV vaccines in the 1960s trials). The winning antibody was about 50 times more potent than the only existing FDA-approved antibody against RSV. The researchers then used a recently discovered human antibody that strongly resembled the mouse antibody to determine the prefusion structure of the F protein and how to chemically keep it in that form.
“After we had that structure, everything really fell in place,” Graham says. “All of a sudden, we had a new, very vulnerable target on the virus for making a vaccine.”
His team spent the next three years growing cells that would produce the prefusion protein and learning how to purify it. The first phase 1 trials began in 2017 and produced encouraging results two years later.
By then “RSV vaccines had a life of their own,” Graham says, as the pharmaceutical industry took over their development. McLellan, meanwhile, turned his focus to coronaviruses. The RSV work would ultimately pave the way for determining the spike protein structure of SARS-CoV-2, the virus that causes COVID, and enable Moderna, Pfizer and other companies to develop a COVID vaccine in record time. The era of protein-structure-based vaccine design—starting with figuring out a pathogen’s protein structure and building a vaccine around it—had begun.
Vaccines Now on the Horizon
The fruits of that labor are now becoming evident as the FDA has begun reviewing several applications for RSV prophylactic products. At the moment, there are only two ways to prevent RSV: the usual hygiene practices used to prevent common colds (such as mask wearing, hand washing and avoiding sick people) and palivizumab, a short-acting monoclonal antibody that provides passive immunity to infants for up to one month at a time. Passive immunity means protection by antibodies created outside of an individual’s own body, whether from a drug such as palivizumab or from antibodies transferred from a pregnant person to a fetus during pregnancy.
But palivizumab is expensive, costing about $1,844 a dose in the U.S. And it requires multiple doses because each one only lasts a month (a typical RSV season lasts five to six months). Although the drug is licensed for preterm infants born before 35 weeks who are under six months old at the start of the RSV season, cost-effectiveness studies have led the American Academy of Pediatrics to recommend restricting the antibody’s use to the most vulnerable of these infants.
In March 2022 AstraZeneca and Sanofi announced that their long-acting antibody, nirsevimab, is 75 percent effective against cases of RSV that require medical care in infants younger than one year old with no history of RSV—and the protection lasts five months. A similar long-acting monoclonal antibody made by Merck, clesrovimab, is in phase 3 trials.
News from several vaccine trials quickly followed the March 2022 announcement. Pfizer announced in August that its single-dose vaccine, now green-lit by VRBPAC, is 86 percent effective against severe disease with at least three symptoms and 67 percent effective against symptomatic disease (illness with at least two symptoms) in adults age 60 and older. Pfizer also announced last November that its maternal RSV vaccine—intended for administration during pregnancy so that maternal antibodies provide passive immunity—is 82 percent effective against severe RSV in newborns for up to three months and 69 percent effective through six months. Pfizer’s vaccine, awaiting FDA priority review, is the only one for pregnant adults moving forward since GSK stopped its maternal vaccine trial because of unspecified safety concerns.
GSK announced last October that its vaccine is 94 percent effective against severe disease and 83 percent effective against symptomatic disease in adults age 60 and older. Most recently, Moderna announced in January that its mRNA-based RSV vaccine is 84 percent effective against symptomatic disease in adults age 60 and older. Two other vaccines for older adults, made by Bavarian Nordic and Janssen, are in phase 3 trials.
None of these vaccines are for newborns, but Karron points out few newborn vaccines exist anyway. And two of these interventions, the antibody nirsevimab and Pfizer’s maternal vaccine, can protect infants when they’re most at risk—as long as health officials can determine the most appropriate way to recommend them.
“A baby doesn’t need both to be protected,” Karron says. But it’s unclear what the CDC might recommend when infants could either be protected by a vaccine in pregnancy or by an antibody drug after birth. “Our fragmented health care system does not allow easy interchangeability of these two products that are given by different sets of providers,” Karron says.
Another challenge will be assuring the protection of children in low-income families, who are already more vulnerable to worse outcomes from RSV. The U.S. Vaccines for Children program ensures all eligible children can receive vaccines recommended by the CDC, but that program doesn’t include prophylactic monoclonal antibodies. “The last thing you want to do is exclude babies who qualify for VFC” from the protection of the monoclonal antibody, Karron says.
Still, by the end of 2023, it’s very likely that older adults and infants will have at least one highly effective option to reduce their risk of RSV for the first time in the half-century since scientists began the effort. That possibility means easing the minds of parents such as Smith, who says she would gladly have gotten a maternal vaccine if it had been available. “It was frustrating,” Smith says about her daughter’s bout with RSV, “because there was nothing I could do to protect her.”
Editor’s Note (3/24/23): This article has been updated to clarify Ruth Karron’s comments and the description of what vaccines the U.S. Vaccines for Children program includes. The text had previously been amended on March 23 to correct Karron’s current affiliation.