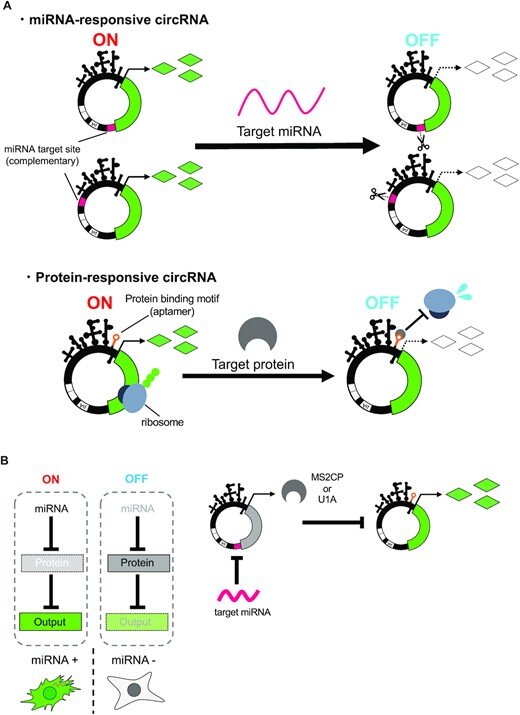
Schematic illustrations of circRNA switches and circuits. (A) Design of miRNA or protein-responsive circRNA switch. miRNA-responsive circRNA has the antisense of target miRNA sequence at the UTR. Protein-responsive circRNA has a protein-binding motif in IRES region. In both systems, gene expression from circRNA is repressed if the target miRNA or protein is present. (B) Scheme of circRNA circuit composed of miRNA- and protein-responsive circRNA switches. The first output (MS2CP or U1A protein) is encoded on a miRNA-responsive circRNA switch, and the second output (reporter protein) is encoded on a protein-responsive circRNA switch. In the OFF state (absence of input miRNAs), MS2CP or U1A protein represses translation of the second output gene-coding circRNA. In ON state (presence of input miRNAs), the MS2CP or U1A translation is repressed by the miRNAs, which leads to output translation. Credit: Nucleic Acids Research (2023). DOI: 10.1093/nar/gkac1252
The Hirohide Saito Laboratory has developed cyclic RNA switches that can control gene expression in a cell type-specific manner using miRNAs and RNA-binding proteins and has successfully constructed an artificial gene circuit by combining them.
Gene transfer technology using synthetic mRNA has the advantages of low risk of genome damage and high transfer efficiency compared to DNA, and thus has a wide range of potential applications, including vaccines, gene therapy, and genome editing. However, RNA is unstable in the cell, making it difficult to sustain gene expression, which is an application challenge.
Because they are not easily degraded in the cells, cyclic RNAs are more stable than linear mRNAs and therefore attracting attention as a new synthetic mRNA that improves RNA persistence. However, specific introduction of mRNA into target cells has been difficult, and unintended protein expression in non-target cells may lead to reduced therapeutic efficacy and side effects in medical applications of mRNA. Therefore, it is necessary to develop a technology to control protein expression (gene expression) from cyclic RNA, but this has not been realized yet.
The Saito laboratory started to work on the development of an RNA switch technology that can control gene expression of cyclic RNAs according to cell type.
It is known that miRNAs induce mRNA degradation and suppress protein synthesis by binding to mRNAs with perfectly complementary RNA sequences in cells. The research group synthesized cyclic RNAs that are intended to respond to endogenous miRNAs to regulate gene expression. When the synthesized miRNA-responsive cyclic RNAs were introduced into cultured cells with a miRNA inhibitor, gene expression was confirmed, but gene expression was suppressed without miRNA inhibitors. This result suggests that the endogenous miRNAs bind to the engineered cyclic RNAs and suppressed its gene expression.
In the same way, they attempted to synthesize cyclic RNAs in which protein-binding motifs were inserted into the internal ribosome entry site (IRES), which enables translation without 5′ cap structure, and gene expression was consequently regulated by the RNA-binding proteins. As a result, they succeeded in constructing the cyclic RNA that can regulate gene expression in an RNA-binding protein-dependent manner without compromising IRES function.
Finally, they developed an artificial gene circuit by combining the two types of cyclic RNA switches and verified whether it functions in the cells. As a result, the researchers showed that specific miRNAs can induce gene expression from cyclic RNAs. The group also confirmed that the gene expression was sustained for a longer period of time compared to the artificial gene circuit composed of normal-type linear mRNAs.
It is expected that the newly developed technologies for cyclic RNA switches and artificial gene circuits will expand the range of applications of mRNA medicine and contribute to solving problems for practical use.
The results of this research were published online in Nucleic Acids Research on January 16, 2023.
More information: Shigetoshi Kameda et al, Synthetic circular RNA switches and circuits that control protein expression in mammalian cells, Nucleic Acids Research (2023). DOI: 10.1093/nar/gkac1252
Provided by Kyoto University
Citation: Cyclic RNA switches that regulate gene expression in a cell type-specific manner (2023, February 16) retrieved 7 March 2023 from https://phys.org/news/2023-02-cyclic-rna-gene-cell-type-specific.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.