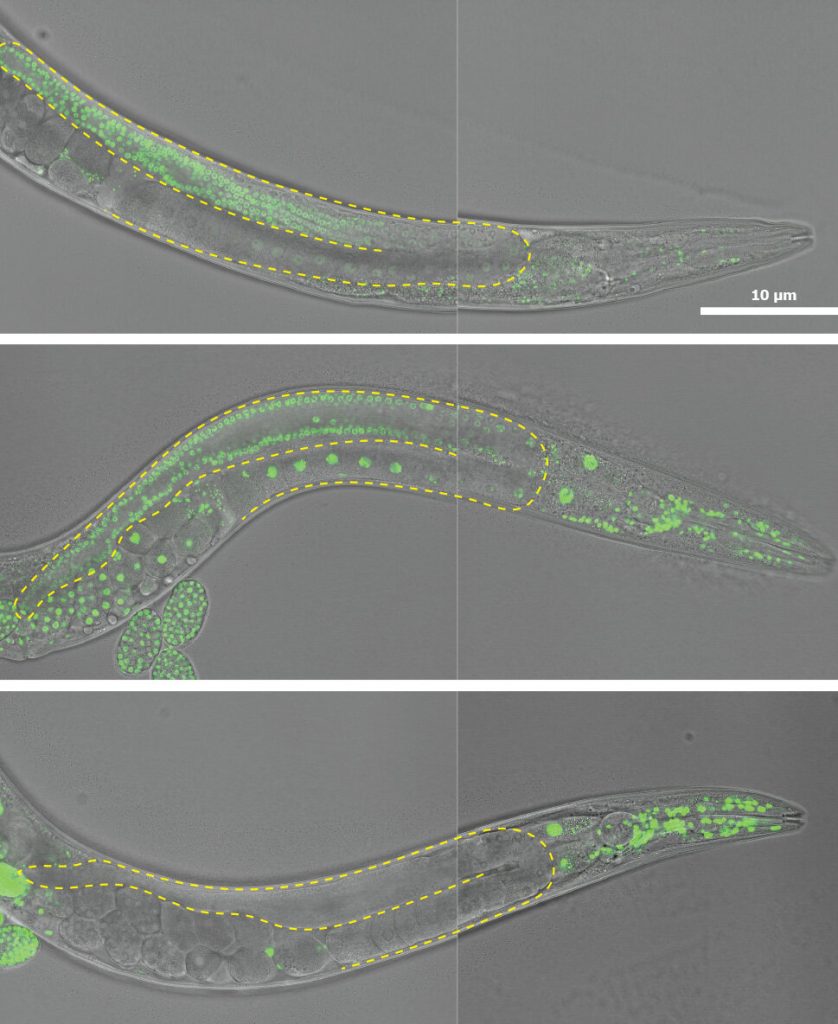
Representative differential interference contrast (DIC) image micrographs of the CRISPR-tagged histones under study. The dashed lines outline the gonads. Credit: Ryan J. Gleason, Johns Hopkins University.
Researchers have spotted how specific proteins within the chromosomes of roundworms enable their offspring to produce specialized cells generations later, a startling finding that upends classical thinking that hereditary information for cell differentiation is mostly ingrained within DNA and other genetic factors.
The Johns Hopkins University team reports for the first time the mechanisms by which a protein known as histone H3 controls when and how worm embryos produce both highly specific cells and pluripotent cells, cells that can turn certain genes on and off to produce varying kinds of body tissue. The details are published today in Science Advances.
The new research could shed light on how mutations associated with these proteins influence various diseases. In children and young adults, for example, histone H3 is closely associated with various cancers.
“These mutations are highly prevalent in different cancers, so understanding their normal role in regulating cell fate and potentially differentiation of tissues may help us understand why some of them are more prevalent in certain diseases,” said lead author Ryan J. Gleason, a postdoctoral fellow in biology at Johns Hopkins. “The histones that we’re looking at are some of the most mutated proteins in cancer and other diseases.”
Histones are the building blocks of chromatin, the structural support of chromosomes within a cell’s nucleus. While histone H3 is particularly abundant in multicellular organisms such as plants and animals, unicellular organisms teem with a nearly identical variant of H3. That’s why scientists think the difference in rations of H3 and its variant hold crucial clues in the mystery of why pluripotent cells are so versatile during early development.
A video clip shows cellular changes in the roundworm from a histone H3 variant in red to an H3-rich genome in green. Credit: Ryan J. Gleason, Johns Hopkins University.
The researchers revealed that as C. elegans roundworm embryos grew, increasing H3 levels in their systems restricted the potential or “plasticity” of their pluripotent cells. When the team changed the worm’s genome to lower the amount of H3, they successfully prolonged the window of time for pluripotency that is normally lost in older embryos.
“As cells differentiate, you start to get a hundredfold histone H3 being expressed at that time period, which coincides with that lineage-specific regulation,” Gleason said. “When you lower the amount of H3 during embryogenesis, we were able to change the normal path of development to adopt alternative paths of cell fate.”
In pluripotent cells, histones help switch certain genes on and off to commit to specific cell types, be they neurons, muscles, or other tissue. Highly regulated by histones, genes act as a voice that tell cells how to develop. How quiet or loud a gene is determines a cell’s fate.
The new findings come from the gene-editing technique CRISPR, which helped the team track the role the two histones played as the worm’s offspring developed. CRISPR has made it much easier for scientists in the last decade to study the nuts and bolts of changing genetic material and spot what that does to animal, plant, and microbe traits, Gleason said.
Even though the C. elegans roundworm gives finer insights into how these pluripotent cells evolve, further research is needed to zero in on how histones might also underpin embryogenesis in humans and animals composed of hundreds of types of cells, said Xin Chen, a Johns Hopkins biology professor and co-investigator.
“Even though we are using this small worm to make these discoveries, really this finding should not be specific to one animal,” Chen said. “It’s hard to imagine the findings are only going to be applicable to one histone or one animal but, of course, more research needs to be done.”
More information: Ryan Gleason et al, Developmentally programmed histone H3 expression regulates cellular plasticity at the parental-to-early embryo transition, Science Advances (2023). DOI: 10.1126/sciadv.adh0411. www.science.org/doi/10.1126/sciadv.adh0411
Provided by Johns Hopkins University
Citation: How a worm’s embryonic cells change its development potential (2023, April 7) retrieved 25 April 2023 from https://phys.org/news/2023-04-worm-embryonic-cells-potential.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.